
Professor Regina Beets-Tan is chair of the department of radiology at The Netherlands Cancer Institute in Amsterdam, full professor of radiology at the University of Maastricht and adjunct professor of abdominal and oncological radiology at the University of Southern Denmark. She will present the Wilhelm Conrad Röntgen Honorary Lecture, titled ‘Oncologic imaging: a new beginning has just begun’ at ECR 2019.
She shared a few thoughts with us on the future of her specialty in an interview ahead of the congress.

Prof. Regina Beets-Tan from Amsterdam will talk about future aspects of oncologic imaging in today’s honorary lecture.
ECR Today: You have chosen quite an iconic and broad topic for this honorary lecture. What points will you cover exactly?
Regina Beets-Tan: The audience will get a glimpse of the future of cancer care and the role of imaging. The world of cancer medicine is changing rapidly. Major steps forward have been taken. Advanced imaging and computing technology, screening programmes; these all will result in the early detection of more tumours. Minimally invasive treatment, including interventional therapy, will have an increasingly important role. Targeted therapy, which specifically hits the cancer genes, and immunotherapy, which uses the patient’s own immune system to kill cancer cells, will result in prolonged survival of patients who are in the final stage of metastatic disease. It will be ‘precision medicine’; we do not want to give the wrong treatment to the wrong patient. As advocated by Prof René Bernards, a respected leader in cancer research at the Netherlands Cancer Institute: ‘Within 15 years, cancer will become a chronic disease’. And I believe this is true. This transformation will change the way we will practice oncologic imaging. This will require us to recreate our discipline. With this lecture, I would like to take my young colleagues on a 20-minute journey towards their future. Read more…

CC 718 – Imaging after systemic therapies: the standards, A-187 A. RECIST criteria (Y. Menu)
A short preview of lecture CC 718 ‘Imaging after systemic therapies: the standards’, from the session A-187 ‘A. RECIST criteria’ at ECR 2014, given by Y. Menu from Paris, France.
Watch the whole lecture and many more at http://ipp.myESR.org
Direct link: http://bit.ly/Imaging_after_systemic_…
Friday, March 7, 16:00 – 17:30 / Room Conf. Room M3
Abstract:
The routine practice of oncologic imaging requires standardisation, which means that we need to harmonise technical protocols and agree on the meaning of selected words for the radiological report. The words Response, Progression and Stable disease are precisely defined according to internationally accepted thresholds and criteria. Although the rules are quite simple and rather easy to apply, they are very efficient in the classification of the response to treatment, and therefore for the medical decisions. However, the role of the radiologist is not limited to measurements and calculation. The detection of new lesions may be challenging and requires experience. The differential between cancer progression and complications of the treatment might be very difficult and requires an adequate communication with the referring clinician. Overall, most of the decisions taken by the clinician will be related to imaging results, stressing the importance of adequate protocols and reports.

Watch this session on ECR Live: Thursday, 16:00–17:30, Room E2
Tweet #ECR2014E2 #MS3
Malignant primary bone tumours like osteosarcoma and Ewing’s sarcoma are very serious diseases mainly affecting children and teenagers. General radiologists are not likely to see these patients every day at their practice, but when they do, they must know what they have to do to optimise patient care and improve outcomes. Experts will give instructions and share useful advice during the dedicated Multidisciplinary Session today at the ECR.
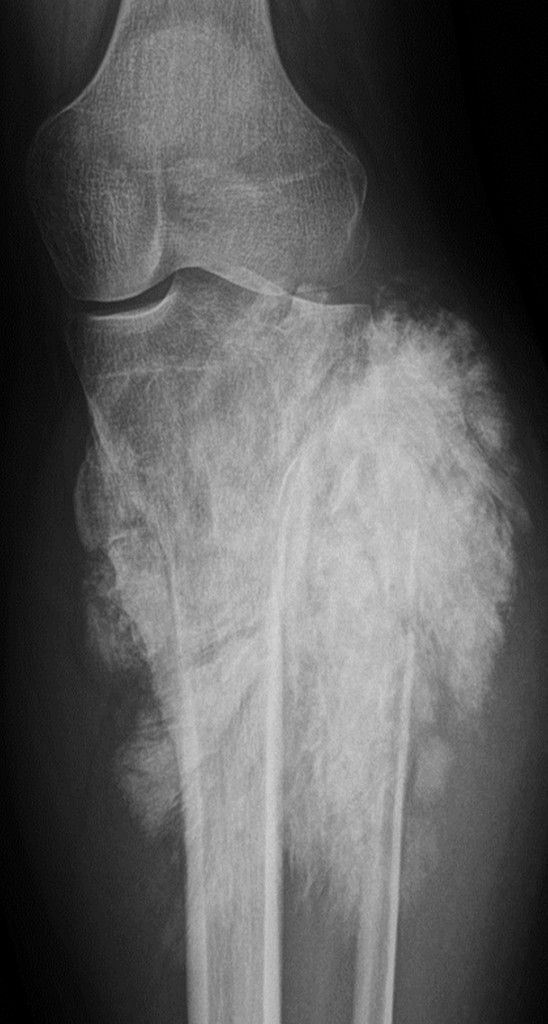
Conventional x-ray of a tumour in the knee
(Image provided by Prof. Koenraad Verstraete)
Read more…

A-545 A. Diagnosis
M. Krokidis | Monday, March 11, 08:30 – 10:00 / Room E1
Oesophageal cancer is the sixth leading cause of death from cancer worldwide. More than 90 % of oesophageal cancers are either squamous-cell carcinomas or adenocarcinomas. Approximately, three quarters of all adenocarcinomas are found in the distal oesophagus, whereas squamous cell carcinomas are more evenly distributed between the middle and lower third. The cervical oesophagus is an uncommon site of disease. The pathogenesis of oesophageal cancer remains unclear. At the time of the diagnosis of oesophageal cancer, more than 50 % of patients have either unresectable tumours or visible metastases on imaging. The most common symptom of presentation is dysphagia which is present in >70% of the cases; odynophagia may also be present in a smaller percentage of patients. The patients are usually presented also with significant weight loss which appears to be also an important prognostic factor of the outcome of the disease. Diagnosis is based on the findings of a contrast swallow- which is usually the first exam to be performed; oesophageal cancer may present as polypoid, infiltrative, varicoid, or ulcerative lesions. Endoscopy usually confirms the findings of the swallow study, revealing the presence of a mass and offering the possibility of taking biopsy samples. Endoscopic ultrasound is the imaging method that is used for local staging and CT and PET-CT are used to determine the presence of metastatic disease. In case of presence of enlarged lymphnodes, fine needle aspiration or even open biopsy may be performed.

A-438 A. The current criteria for nodal involvement on CT/MRI
W. Schima | Sunday, March 10, 14:00 – 15:30 / Room E2
In a variety of diseases, such as metastatic disease, lymphoma and inflammation, lymph node enlargement can be seen. Thus, lymph node characterization is important to differentiate between benign and malignant disease. It is based on size (short axis diameter) and morphologic criteria, such as shape, homogeneity, and contrast enhancement. For abdominal nodes, location-specific size criteria apply (upper limit of normal: lower paraaortic 11 mm, upper paraaortic 9 mm, gastrohepatic ligament 8 mm, portocaval space 10 mm, retrocrural space 6 mm; pelvic nodes 10 mm). However, in clinical practice, often a universal size threshold of 10 mm is used in abdominal imaging. In chest CT, an upper limit of normal of 10 mm is universally applied. However, size criteria alone are unreliable: CT for lung cancer staging has a pooled sensitvity of 51 % (i.e., false negative diagnoses of metastatic deposits in normal-sized nodes), and a specificity of 86 % (i.e., false positive diagnoses due to enlarged reactive nodes). With MRI, the same size criteria apply. However, additionally features such as central necrosis (T2w fatsat or gadolinium-enhanced images) are suggestive of metastasis (or suppurative infection). Lymph node-specific USPIO MR agents can depict tumour deposits in subcentimeter pelvic nodes. Unfortunately, they did not reach market approval. DWI is helpful in identifying in lymph nodes as they exhibit high SI with higher b-values. However, diffusion pattern of benign and malignant nodes overlap, so that ADC values do not aid in characterization. Despite the use of modern MDCT and MRI techniques, lymph node characterization needs further improvement.

B-0801 Treatment response assessment in Hodgkin lymphoma: in search for morphological correlates of metabolic activity
T. Knogler, G. Karanikas, M. Weber, K. El-Rabadi, M.E. Mayerhoefer | Monday, March 11, 10:30 – 12:00 / Room F1
Purpose: To predict early treatment response with three-dimensional texture features (TF) in patients with Hodgkin lymphoma (HL) after radio-chemotherapy extracted from contrast-enhanced CT.
Methods and Materials: 21 patients with histologically proven HL were included in this study. Contrast-enhanced (18)F-FDG PET/CT was obtained on a dedicated PET/CT scanner. Volumes-of-interest and long- and short-axis diameter were manually defined on 48 HL manifestations prior and post-radio-chemotherapy on the CT image stack. Three-dimensional texture features derived from the grey-level histogram, co-occurrence matrix, run-length matrix and absolute gradient were calculated for the VOIs. A stepwise logistic regression with forward selection was performed to find classic radiologic features (i.e. lesion diameter, lesion volume) and TF, which correctly classify treatment response (i.e. full response and partial response). Classification in PET/CT was used as reference.
Results: Difference in short axis diameter best fit as classic feature with a sensitivity of 100 %, a specificity of 54,5% and an accuracy of 89,6%. Combination of “S_0_0_1_Entrp” and difference of “vertl_fraction” best fit as TF with a sensitivity of 97,3%, a specificity of 72,7% and an accuracy of 91,7%.
Conclusion: Texture features extracted from contrast-enhanced CT in patients with HL are superior in differentiation between responders and non-responders without the need for PET examinations, compared to classical radiological features. However, PET/CT as state-of-the-art imaging technique has a sensitivity and specificity >90%, so that further research with larger patient number is needed to investigate this new method.

A-445 Biliary procedures
M. Krokidis, A.A. Hatzidakis | Sunday, March 10, 14:00 – 15:30 / Room F1
Palliative Percutaneous Transhepatic Biliary Drainage (PTBD) is a therapeutic procedure leading to drainage of the obstructed bile duct system. If endoscopy is not possible and if patient is inoperable, then the percutaneous treatment is indicated. Drainage of the bile ducts is performed with a small plastic multiple hole pigtail catheter. Self-locking catheters are preferred in order to minimize the dislocation risk. The percutaneous catheter is pushed through the malignant stricture, so that bile is draining through the catheter towards the bowel loops. Technical success rate of percutaneous biliary drainage can reach nearly 100 % in experienced hands, while the major complications rate is usually lower than 5 %. Clinical efficacy is usually lower, but still over 90 %. The drainage procedure can be extended with the placement of a permanent metallic stent, which keeps the stenosed biliary duct patent, without need for a catheter. Metallic biliary stents have been proved as the best palliative treatment of non-resectable malignant obstructive jaundice, allowing longer patency rates than plastic endoprostheses. The technique is safe, with low-complication rate and procedure-related mortality between 0.8 and 3.4%. Still controversial remains in the timing between initial drainage and metallic stent placement, as well as the question of balloon dilatation before stent insertion. There is evidence that if the initial transhepatic drainage is completed without causing any severe complications, especially bleeding in form of haemobilia, primary metallic stenting can follow as a single-step procedure.

B-0976 Can a contrast-enhanced ultrasound nephrostogram be used instead of a fluoroscopic nephrostogram: preliminary findings
M. Daneshi, K. Patel, D. Huang, M. Sellars, P. Sidhu | Monday, March 11, 14:00 – 15:30 / Room G/H
Purpose: The use of contrast-enhanced ultrasound (CEUS) has extended beyond traditional uses, and the possibility to delineate percutaneous tubes and drains is achievable. We have compared the traditional fluoroscopic nephrostogram using iodinated contrast agents with CEUS nephrostogram to ascertain the accuracy, utility and convenience of the CEUS nephrostogram.
Methods and Materials: The standard conventional nephrostogram was performed immediately prior to the CEUS nephrostogram. The CEUS nephrostogram technique involved diluting 0.2ml of SonoVue with 40 ml of normal saline and introduced into the renal collecting system via the nephrostomy tube. Digital cine-clips and still images were recorded to allow accurate retrospective comparison by two independent reviewers to the reference standard.
Results: Twelve nephrostomies in 10 patients (median age 64 yrs, range 29-91 yrs, 6 females and 4 males) were performed and reviewed. The renal pelvicalyceal system was visualised in both CEUS and fluoroscopic nephrostograms in 11/12 (92%) with one nephrostomy tube identified as being misplaced. The entire ureter was visualised in 6/12 (50%) with a CEUS nephrostogram compared with 8/12 (75%) using traditional nephrostogram. Fluoroscopic nephrostogram showed drainage of contrast into the bladder in 10/12 (83%) cases compared with 9/12 (75%) using CEUS.
Conclusion: Preliminary results suggest that CEUS nephrostogram is a feasible method to confirm the correct positioning of the nephrostomy tube, image the ureters and determine if there is satisfactory drainage into the bladder. CEUS nephrostogram is a suitable alternative for the traditional nephrostogram in patients with contraindications to iodinated contrast agents or if the procedure needs to be performed at the bedside.

A-549 A. Head and neck cancer
L. Oleaga Zufiría | Monday, March 11, 08:30 – 10:00 / Room E2
Identification of recurrent tumour in the post-therapy setting is often challenging. It is essential to have a baseline study for monitoring changes and evaluating possible recurrences. After surgery, there is distortion of the anatomy, the fat planes are lost, functional lymphadenectomy may be associated with muscle resection, resection of the jugular vein or grafting, therefore, it is essential to know this data to properly analyze the images. The immediate postoperative baseline study after surgery should be performed between 4 and 6 weeks after surgery. When radiotherapy is performed, there is loss of fat planes, significant edema in the mucosa and in subcutaneous tissue. The recommended baseline study should be obtained at 3 months after a completion of radiation therapy. The imaging methods used for the staging and follow-up of head and neck tumours varies between centres. CT is the most commonly used imaging technique, however, at some institutions MRI or PET are used to detect tumour recurrence after therapy, either surgery or chemo/radiation. Distinction between post-treatment changes and recurrent or residual tumor might be difficult to assess on imaging. A soft tissue mass present in the baseline study that decreases in size, should be considered treatment-related changes. If the mass increases in size, it is suggestive of persistent or recurrent tumor. A new onset mass in the follow-up study should be considered as recurrence. Late complications due to chemo/radiation include soft-tissue necrosis, osteochondronecrosis, carotid atherosclerosis, myelopathy, nerve paralysis secondary to fibrosis and sarcomas.

MS 4 – Hepatocellular carcinoma
B. Sangro, A. Benito, J.I. Bilbao, F. Pardo | Friday, March 8, 08:30 – 10:00 / Room F1
A-075 Chairman’s introduction
A variety of options are available for the treatment of hepatocellular carcinoma (HCC) from liver transplantation or resection to percutaneous ablation by chemical or physical procedures, intraarterial injection of embolizing particles that may also serve as carriers of chemotherapeutic agents or radiation-emitting isotopes, or systemic delivery of molecularly targeted agents. Although large scale studies have identified groups of patients that may certainly benefit from some of these therapeutic tools, many areas of uncertainty still exist. Only by the coordinated action of HPB Oncology multidisciplinary teams may patients with HCC receive the best possible treatment.
Read more…











